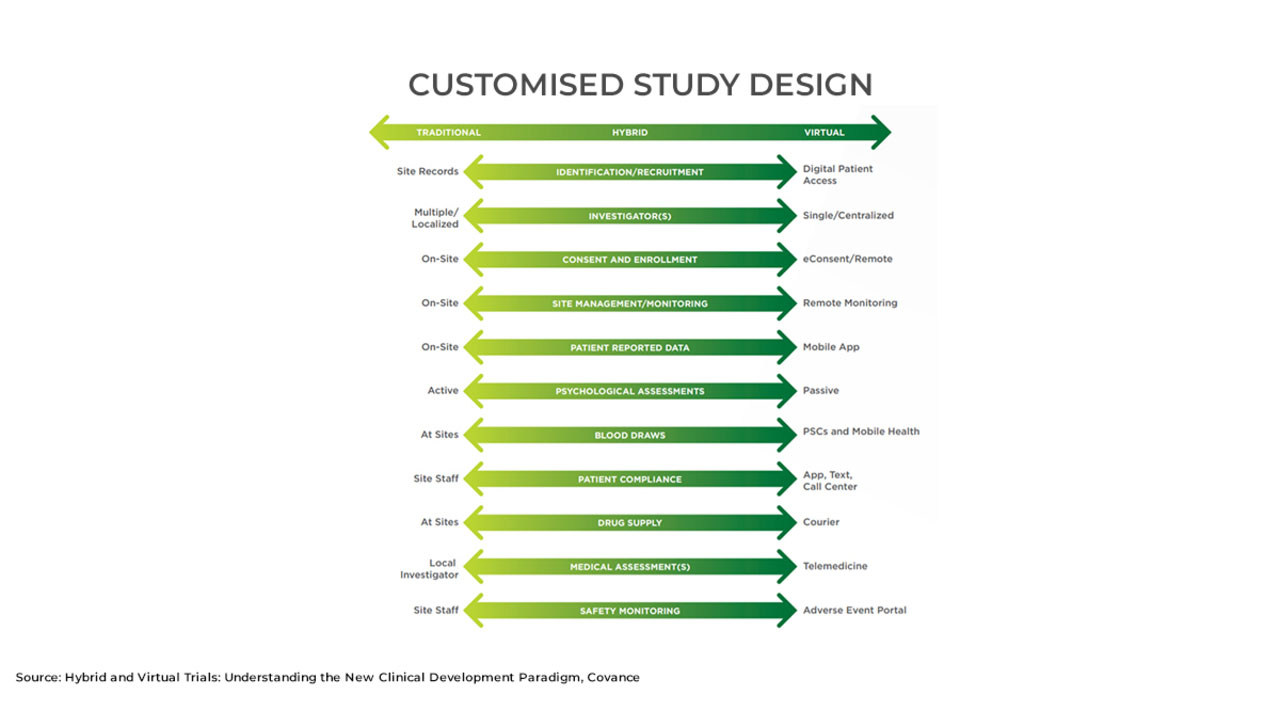
Clinical Trials and Remote Patient Monitoring
According to a BCG Physician COVID-19 Response Biopharma Survey, patient-doctor interaction using telemedicine has soared.
- Pre-COVID, the percentage of physicians interacting with patients via telemedicine was just 25.
- During COVID, the figure spiked to 72% and post-COVID, it was a solid 65%.
Why Virtual Clinical Trials
How Technology Is Enhancing Patient Experience
- Wide reach: Virtual clinical trials allow for a wider geographical reach and a diverse patient population. People living in rural and remote regions can participate, which enables researchers to gather comprehensive data. Participant recruitment is also faster, resulting in quicker product test and launch.
- Lower costs: Clinical trials are conducted over three phases. Phase I, which is the smallest, involves monitoring how well a drug is tolerated in around 20 to 100 volunteers. Once completed, the trial moves to Phase II, which involves a few hundred volunteers. Here, researchers determine how well the drug works and monitor its side effects. In Phase III, the trial takes place longer and the participant pool increases. Volunteers are split into two groups where one receives a placebo and the other receives the drug.
Phase II and III are the most expensive with most of the cost channeled into a central clinic that participants must report to. Often, participants do not complete the trial owing to travel costs and an unwillingness to visit the clinic frequently. This prolongs the trials and reduces participant diversity. The drug’s safety is thereby impacted as subsets of populations differ from one another.
Virtual clinical trials can help reduce overall cost by allowing participants to complete the trials at home so they do not need to visit a central clinic. Using wearable devices and smartphone apps, researchers can monitor participants’ responses. Participants, in turn, have quick access to investigators and healthcare providers. They can ask questions and are likelier to comply with trial requirements.
- Increased data quality and shareability: Virtual clinical trials facilitate larger volunteer participation, which increases the quality of data collected. The majority of data is automatically transmitted to a clinical database instead of relying on staff to manually enter information in the database. This same data can be shared between investigators to strengthen collaboration. Virtual clinical trials also remove tedious administrative tasks and leave investigators free to perform important work.
- More patient-centric: Virtual clinical trials allow patients – including those with mobility issues – to participate in tests from the comfort of their homes. Being in a safe environment encourages greater participation, which leads to better engagement. Patients become more eager to participate in long-term trials too so there are fewer drop-outs.
Technologies and Processes to Enhance Clinical Trials
A variety of modern technologies and processes can enhance patient enrollment rates. These include:
RPM: Remote patient monitoring allows investigators and healthcare providers to monitor patients outside clinical settings. It also enables patients to remain independent and helps them manage self-care processes.
AI and predictive analysis: Artificial Intelligence (AI) can analyze data, make assumptions and provide predictions to optimize study delivery and reduce clinical trial design risks. It can also quickly identify people who are suitable for recruitment.
Precision medicine: With data such as environment, genetics and lifestyle collected through telemedicine, healthcare providers can customize treatment and products for patients. This edges out a one-size-fits-all solution, which is not effective and may have serious side effects.
Direct-to-patient recruitment: Rather than rely on investigatory sites to provide access to patients, social media can connect pharma companies with specific demographics in chosen cities. Another way to find patients is through patient advocacy groups, which are organizations that provide knowledge, emotional support and material sources to those in need. They also act as a bridge between patients and pharma companies.
For pharma companies, remote health monitoring provides novel ways to capture valuable real-time data and engage with health providers to improve care decisions and outcomes. On the other hand, patients have the benefit of easy access to care and learning to manage their own health remotely. They become better informed and more responsible, which also reduces patient drop-out rates at clinical trials as well as hospital admissions and readmissions.